Levothyroxine Brillpharma 50 microgram/5 ml Oral Solution
Therapeutic indications include:
- Hypothyroidism (congenital or acquired)
- Diffuse non-toxic goitre
- Goitre associated with Hashimoto’s thyroiditis
- Suppression therapy in thyroid carcinoma1
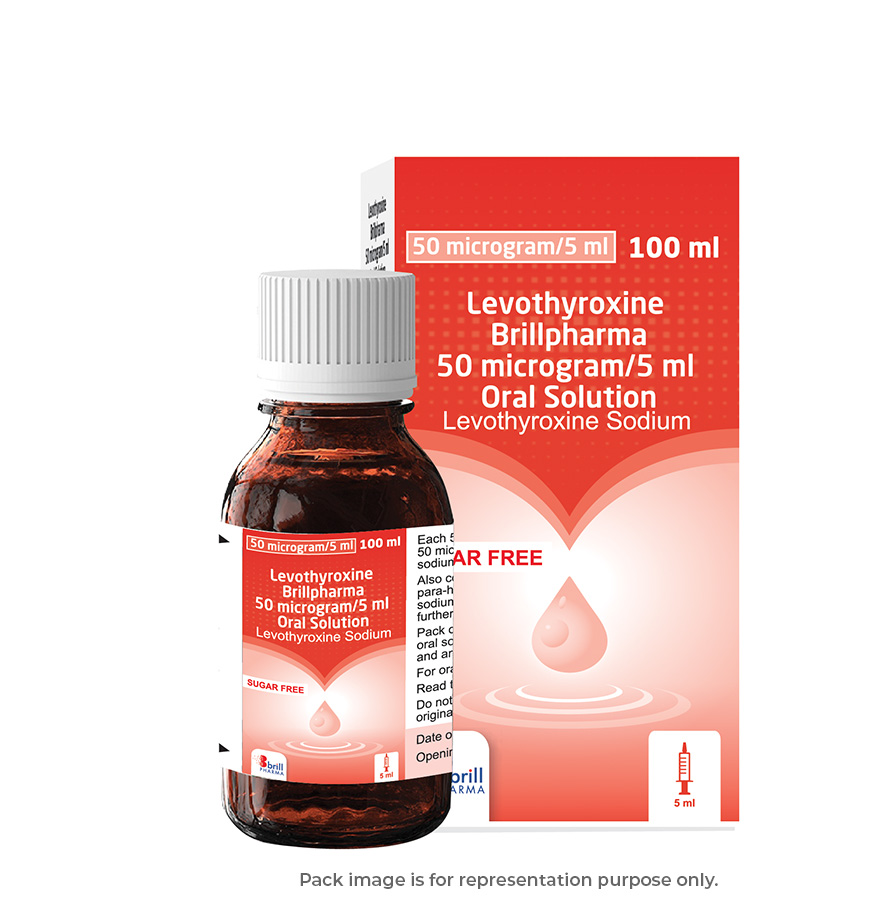
| Strength | 50 microgram/5ml |
| Pack Size | 100 ml |
| Measuring Device | 5 ml oral syringe (graduated at every 0.1 ml) and an adaptor for the syringe. |
| Regulatory Status | POM |
| Flavour | – |
| Suitable for Vegetarians | Yes |
| GTIN | 5060712420096 |
| PIP code | 125-0125 |
Product information and Risk materials
| Summary of Product Characteristics (SPC) | Download |
| Patient Leaflet (PIL) | – |
| Risk Materials | – |

Manufacturing
Our quality and safety teams monitor and analyse each stage of the production and distribution process to make sure our products are of the highest quality. The application of Good Manufacturing Practice (GMP) means that we have the appropriate standards and procedures in place to help us achieve this.
How to report adverse events
Adverse events should be reported.
If you are based in the UK, reporting forms and information can be found at: https://yellowcard.mhra.gov.uk.
Adverse events should also be reported to Brill Pharma
Tel: 0044 (0) 1582 545500 Email: info@brillpharma.co.uk
If you are based outside of the UK, please report adverse events to Brill Pharma using the contact details provided above.
Have a question?
If you have a question about Brill Pharma or any products in our range please get in touch
Tel: 0044 (0) 1582 545500 Email: info@brillpharma.co.uk
